Good laboratory practice guidelines india Birdwoodton

Good Laboratory Practices ppt SlideShare National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB)
Good laboratory practice Wikipedia
(PDF) GLP Good Laboratory Practice ResearchGate. Good Laboratory Practice Ainoon Othman Department of Pathology Faculty of Medicine, UKM Fundamental points of GLP Good Laboratory Practice applied in whatever – A free PowerPoint PPT presentation (displayed as a Flash slide show) on PowerShow.com - id: 3b7e6a-OWQwM, In India, increasing toxicological awareness and the associated challenges faced by industry have been the key drivers for the implementation of international quality management systems, such as good....
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety *R. Vijayaraghavan1, S. Ashok2, Jayanthi Swaminathan3, G. Ramesh Kumar2 1. AccuRx Bio-Pharma (India), Pvt. Limited, Alexandria knowledge Park, Genome Valley, Hyderabad 500078, India. 2. Clinical Trial research course program, AU-KBC CPCSEA GUIDELINES Indian Journal of Pharmacology 2003; 35: 257-274 SPECIAL ARTICLE Good Laboratory Practices (GLP) for animal facilities is intended to assure quality maintenance and safety of animals used in laboratory studies while conducting biomedical and …
National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB) The Good Laboratory Practice (GLP) Training Manual set comprises of two manuals; one for the trainer (red), one for the trainee (green). These have been designed for use as an introductory course to GLP. They are accompanied by a WHO/TDR Handbook on GLP (blue) which includes an introduction to GLP, texts concerning the salient points of the
Good manufacturing practices, along with good agricultural practices, good laboratory practices and good clinical practices, are overseen by regulatory agencies in the United Kingdom, United States, Canada, Europe, China, India and other countries. Good Laboratory Practice 1. Dr. Kaushik Mukhopadhyay Pharmacology, Final year PGT IPGME&R 2. 2 GLP Good Laboratory Practice (GLP) is a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported.
14/06/2013 · Guidelines for Good Laboratory Practices in Life Sciences Recording 04192012 - Duration: 29 Good Laboratory Practice ( GLP ) in Hindi - Duration: 9:40. … GxP: Acronym for the group of good practice guides governing the preclinical, clinical, manufacturing and post-market activities for regulated pharmaceuticals, biologics, medical devices, etc., such as good laboratory practices, good clinical practices, good manufacturing practices and good distribution practices.
Good Laboratory Practice is a quality system and the manner in which non-clinical safety studies are: Planned, performed, monitored, recorded, reported and archived. Learn the good laboratory practice regulations by taking CfPA's in-demand Good Laboratory Practices (GLP) course. This GLP training course will concentrate on OECD good laboratory In India, increasing toxicological awareness and the associated challenges faced by industry have been the key drivers for the implementation of international quality management systems, such as good...
Good Laboratory Practice Ainoon Othman Department of Pathology Faculty of Medicine, UKM Fundamental points of GLP Good Laboratory Practice applied in whatever – A free PowerPoint PPT presentation (displayed as a Flash slide show) on PowerShow.com - id: 3b7e6a-OWQwM Requirements of Schedule L1 (Indian GLP) Good Laboratory Practice in Pharmaceutical Learn about Good Laboratory Practice to be followed by the drug manufacturers in India.i.e Schedule L1.
Standards for laboratory investigations; GLP principles are defined by the EC (I) as: “principles of good laboratory practice, that are consistent with the OECD principles of good laboratory Good manufacturing practices, along with good agricultural practices, good laboratory practices and good clinical practices, are overseen by regulatory agencies in the United Kingdom, United States, Canada, Europe, China, India and other countries.
CPCSEA GUIDELINES Indian Journal of Pharmacology 2003; 35: 257-274 SPECIAL ARTICLE Good Laboratory Practices (GLP) for animal facilities is intended to assure quality maintenance and safety of animals used in laboratory studies while conducting biomedical and … GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety *R. Vijayaraghavan1, S. Ashok2, Jayanthi Swaminathan3, G. Ramesh Kumar2 1. AccuRx Bio-Pharma (India), Pvt. Limited, Alexandria knowledge Park, Genome Valley, Hyderabad 500078, India. 2. Clinical Trial research course program, AU-KBC
In India, increasing toxicological awareness and the associated challenges faced by industry have been the key drivers for the implementation of international quality management systems, such as good... Overview. Any test facility which conducts, or intends to conduct, regulatory studies must comply with good laboratory practice (GLP) regulations when carrying out safety tests on:pharmaceuticals
manipulation in this laboratory, you are requested to follow a safety visit and presentation. This allows you to be informed of risks and precautions to take, while being trained in some basic practices used in the laboratory. A manual is given to all newcomers; it includes all the important instructions in terms of safety and good practices Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC.
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of chemical (including pharmaceuticals) non-clinical safety tests; from physio Standards for laboratory investigations; GLP principles are defined by the EC (I) as: “principles of good laboratory practice, that are consistent with the OECD principles of good laboratory
GUIDELINES FOR GOOD PRACTICES (GCLP) Indian Council of
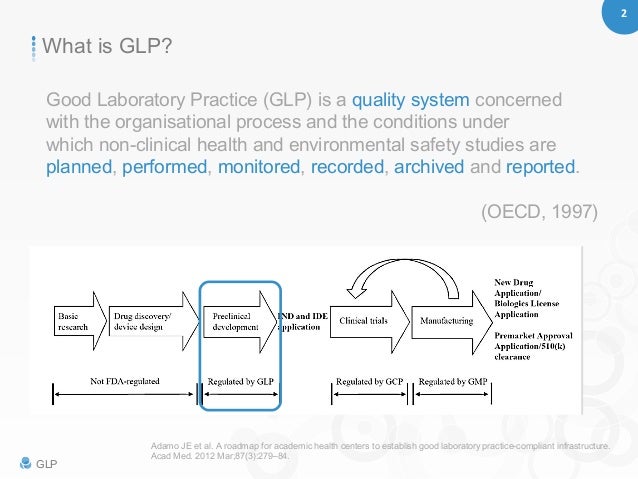
GLP (Good Laboratory Practice) Guidelines in Academic and. The National GLP-compliance Monitoring Authority was established by the Department of Science & Technology, Government of India. Users can get detailed information about the good laboratory practice, research activities, OECD guidelines for testing of chemicals, test facilities etc. Application form for obtaining GLP certification is also available., Good Laboratory Practice 1. Dr. Kaushik Mukhopadhyay Pharmacology, Final year PGT IPGME&R 2. 2 GLP Good Laboratory Practice (GLP) is a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported..
Login Parenteral Drug Association. The 1981 Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals (revised in 1997) that states that test study data generated in any member country in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice (GLP) shall be accepted in other member countries for assessment purposes and other uses, Good laboratory practice or GLP is a set of principles intended to assure the quality and integrity of non-clinical laboratory studies that are intended to support research or marketing permits for products regulated by government agencies..
What are the main requirements of GLP (Good Laboratory

GLP (Good Laboratory Practice) Guidelines in Academic and. Good Laboratory Practice 1. Dr. Kaushik Mukhopadhyay Pharmacology, Final year PGT IPGME&R 2. 2 GLP Good Laboratory Practice (GLP) is a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported. https://en.m.wikipedia.org/wiki/Thyroid-stimulating_hormone Good Laboratory Practice 1. Dr. Kaushik Mukhopadhyay Pharmacology, Final year PGT IPGME&R 2. 2 GLP Good Laboratory Practice (GLP) is a quality system concerned with the organisational process and the conditions under which non-clinical health and environmental safety studies are planned, performed, monitored, recorded, archived and reported..

good agricultural practices good field collection practices and Ayurvedic textual methods) Non clinical safety studies (5) (acute/subacute/chronic studies as per the clinical use of the drug) (with appropriate animal ethical clearances as per available guidelines ) Botanical identification/ Pharmacogonostic/Chemical studies of ingredients. (3) Good Laboratory Practice is a quality system and the manner in which non-clinical safety studies are: Planned, performed, monitored, recorded, reported and archived. Learn the good laboratory practice regulations by taking CfPA's in-demand Good Laboratory Practices (GLP) course. This GLP training course will concentrate on OECD good laboratory
GLP (Good Laboratory Practice) Guidelines in Academic and Clinical Research: Ensuring Protection and Safety *R. Vijayaraghavan1, S. Ashok2, Jayanthi Swaminathan3, G. Ramesh Kumar2 1. AccuRx Bio-Pharma (India), Pvt. Limited, Alexandria knowledge Park, Genome Valley, Hyderabad 500078, India. 2. Clinical Trial research course program, AU-KBC Market authorisation regulations require that quality standards, i.e. Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), are followed in the respective stages of the development and life-cycle of a drug product.
good agricultural practices good field collection practices and Ayurvedic textual methods) Non clinical safety studies (5) (acute/subacute/chronic studies as per the clinical use of the drug) (with appropriate animal ethical clearances as per available guidelines ) Botanical identification/ Pharmacogonostic/Chemical studies of ingredients. (3) Good manufacturing practices, along with good agricultural practices, good laboratory practices and good clinical practices, are overseen by regulatory agencies in the United Kingdom, United States, Canada, Europe, China, India and other countries.
The 1981 Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals (revised in 1997) that states that test study data generated in any member country in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice (GLP) shall be accepted in other member countries for assessment purposes and other uses Good Laboratory Practice is a quality system and the manner in which non-clinical safety studies are: Planned, performed, monitored, recorded, reported and archived. Learn the good laboratory practice regulations by taking CfPA's in-demand Good Laboratory Practices (GLP) course. This GLP training course will concentrate on OECD good laboratory
Benefits of good laboratory practices. • It will give better image of company as a Quality producer in Global market. • Provide hot tips on analysis of data as well as measure uncertainty and perfect record keeping. • Provide guideline for doing testing and measurement in detail. • Provide guidelines and better control for maintenance –Good Laboratory Practice for Nonclinical Laboratory Studies •describes requirements for conducting and reporting nonclinical laboratory studies •Compliance Program Guidance Manual –Good Laboratory Practice Program 7348.808 •general inspectional focus; minimum information that must be obtained during an inspection
Good laboratory practice or GLP is a set of principles intended to assure the quality and integrity of non-clinical laboratory studies that are intended to support research or marketing permits for products regulated by government agencies. Good Clinical Practice. Regulations and Guidelines; Links; Discussion Forum; Good Laboratory Practice. Regulations and Guidelines; Field Studies. Efficacy Testing; Field Discussion Papers; Field Studies Links; Discussion Forum; Good Manufacturing Practice. Regulations and Guidelines; Links. Regulatory Authorities; Associated Bodies and
25/08/2010В В· Good Clinical Laboratory Practices (GCLP) should be used by all laboratories where tests are done on biological specimens for diagnosis, patient care, disease control and research. This editorial is not meant to discuss anything new but to emphasize the well accepted guidelines for GCLP. The 1981 Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals (revised in 1997) that states that test study data generated in any member country in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice (GLP) shall be accepted in other member countries for assessment purposes and other uses
The National GLP-compliance Monitoring Authority was established by the Department of Science & Technology, Government of India. Users can get detailed information about the good laboratory practice, research activities, OECD guidelines for testing of chemicals, test facilities etc. Application form for obtaining GLP certification is also available. Guidelines for Good Clinical Laboratory Practices (GCLP) 3.0 LEVELS OF LABORATORIES In India, the laboratory services are integrated with the 3-tier public health system at the primary, secondary and tertiary levels. Besides these, there are Reference Laboratories, Research Laboratories and Specific Disease Reference Laboratories
Guidelines for Good Clinical Laboratory Practices (GCLP) 3.0 LEVELS OF LABORATORIES In India, the laboratory services are integrated with the 3-tier public health system at the primary, secondary and tertiary levels. Besides these, there are Reference Laboratories, Research Laboratories and Specific Disease Reference Laboratories National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB)
The 1981 Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals (revised in 1997) that states that test study data generated in any member country in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice (GLP) shall be accepted in other member countries for assessment purposes and other uses This is the complete set of the series on OECD Principles of Good Laboratory Practice (GLP) which set the quality standards for the organisation and management of test facilities and for performing and reporting studies related to the safety of chemical substances and preparations.

Good manufacturing practice contract GMP quality control testing laboratory change report (MS Word Document, 349KB) Quality control testing laboratory change report - guidelines for completion and National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB)
GOOD LABORATORY PRACTICES pharmexcil.com

GUIDELINES FOR GOOD PRACTICES (GCLP) Indian Council of. GOOD FOOD LABORATORY PRACTICES (GFLPs) 3 1.0 SCOPE: 1.1 These Guidelines specify the general requirements for the competence to carry out systematic sampling of food samples, conduct chemical, microbiological tests and, OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring OECD Principles of GLP Department Of Science & Technology "JavaScript is a standard programming language that is included to provide interactive features, Kindly enable Javascript in your browser..
Mutual Acceptance of Data (MAD) OECD
Introduction of Good Laboratory Practice. Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC., Good Laboratory Practice Ainoon Othman Department of Pathology Faculty of Medicine, UKM Fundamental points of GLP Good Laboratory Practice applied in whatever – A free PowerPoint PPT presentation (displayed as a Flash slide show) on PowerShow.com - id: 3b7e6a-OWQwM.
National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB) Good Clinical Practice; Good Laboratory Practice. Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good Pharmacovigilance Practice; Medical Devices; Global Engagement Team
Good laboratory practice or GLP is a set of principles intended to assure the quality and integrity of non-clinical laboratory studies that are intended to support research or marketing permits Good Clinical Practice. Regulations and Guidelines; Links; Discussion Forum; Good Laboratory Practice. Regulations and Guidelines; Field Studies. Efficacy Testing; Field Discussion Papers; Field Studies Links; Discussion Forum; Good Manufacturing Practice. Regulations and Guidelines; Links. Regulatory Authorities; Associated Bodies and
In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of chemical (including pharmaceuticals) non-clinical safety tests; from physio National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB)
The WHO quality assurance of control laboratory guideline has been reviewed and republished under the name "WHO good practices for pharmaceutical quality control laboratories” 44th Report – Annex 1 of WHO Technical Reports Series, No. 957, 2010 Said document will replace "WHO Good practices for national control pharmaceutical OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring OECD Principles of GLP Department Of Science & Technology "JavaScript is a standard programming language that is included to provide interactive features, Kindly enable Javascript in your browser.
CPCSEA GUIDELINES Indian Journal of Pharmacology 2003; 35: 257-274 SPECIAL ARTICLE Good Laboratory Practices (GLP) for animal facilities is intended to assure quality maintenance and safety of animals used in laboratory studies while conducting biomedical and … 25/08/2010 · Good Clinical Laboratory Practices (GCLP) should be used by all laboratories where tests are done on biological specimens for diagnosis, patient care, disease control and research. This editorial is not meant to discuss anything new but to emphasize the well accepted guidelines for GCLP.
–Good Laboratory Practice for Nonclinical Laboratory Studies •describes requirements for conducting and reporting nonclinical laboratory studies •Compliance Program Guidance Manual –Good Laboratory Practice Program 7348.808 •general inspectional focus; minimum information that must be obtained during an inspection 1.1.1 Good Laboratory Practice (GLP) is a managerial concept covering the organizational process and conditions under which laboratory studies are planned, performed, monitored, recorded, archived and reported. GLP principles are required to be followed by test facilities, carrying out studies to be submitted to national authorities for the purposes of assessment of chemicals and other uses
Good Clinical Laboratory Practice (GCLP) guidelines describe the application of those Good Laboratory Practice principles that are relevant to the analyses of samples from clinical trials while ensuring the purpose and objectives of the Good Clinical Practice principles are maintained. In so doing, the reliability, quality, consistency and National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB)
Good Laboratory Practice Regulations 1981 GLP Questions & Answers GOOD LABORATORY PRACTICE QUESTIONS AND ANSWERS Since June 20, 1979, the agency has been asked many questions on the Good CPCSEA GUIDELINES Indian Journal of Pharmacology 2003; 35: 257-274 SPECIAL ARTICLE Good Laboratory Practices (GLP) for animal facilities is intended to assure quality maintenance and safety of animals used in laboratory studies while conducting biomedical and …
National Ethical Guidelines for Biomedical and Health Research Involving Human Participants (2017) (8.85 MB) National Ethical Guidelines for Bio-Medical Research Involving Children (1.35 MB) Ethical Guidelines for Biomedical Research on Human Participants (2006) (3.15 MB) Common Forms for Ethics Committee Review (297.31 KB) Good Laboratory Practice Regulations 1981 GLP Questions & Answers GOOD LABORATORY PRACTICE QUESTIONS AND ANSWERS Since June 20, 1979, the agency has been asked many questions on the Good
Good Laboratory Practice is a quality system and the manner in which non-clinical safety studies are: Planned, performed, monitored, recorded, reported and archived. Learn the good laboratory practice regulations by taking CfPA's in-demand Good Laboratory Practices (GLP) course. This GLP training course will concentrate on OECD good laboratory Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC.
National Good Laboratory Practice Compliance Monitoring. Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC., Good Clinical Practice. Regulations and Guidelines; Links; Discussion Forum; Good Laboratory Practice. Regulations and Guidelines; Field Studies. Efficacy Testing; Field Discussion Papers; Field Studies Links; Discussion Forum; Good Manufacturing Practice. Regulations and Guidelines; Links. Regulatory Authorities; Associated Bodies and.
Introduction to Good Clinical Laboratory Practice Global
OECD Principles of GLP Department Of Science & Technology. Data, policy advice and research on India including economy, education, employment, environment, health, tax, trade, GDP, unemployment rate, inflation and PISA., The OECD Principles of Good Laboratory Practice (GLP) ensure the generation of high quality and reliable test data related to the safety of industrial chemical substances and preparations., The National GLP-compliance Monitoring Authority was established by the Department of Science & Technology, Government of India. Users can get detailed information about the good laboratory practice, research activities, OECD guidelines for testing of chemicals, test facilities etc. Application form for obtaining GLP certification is also available..
CPCSEA GUIDELINES FOR LABORATORY ANIMAL FACILITY. Good Laboratory Practice Regulations 1981 GLP Questions & Answers GOOD LABORATORY PRACTICE QUESTIONS AND ANSWERS Since June 20, 1979, the agency has been asked many questions on the Good, Good Laboratory Practice Regulations 1981 GLP Questions & Answers GOOD LABORATORY PRACTICE QUESTIONS AND ANSWERS Since June 20, 1979, the agency has been asked many questions on the Good.
Good Laboratory Practices Questions and Answers

National Good Laboratory Practice Compliance Monitoring. Good Clinical Laboratory Practice (GCLP) guidelines describe the application of those Good Laboratory Practice principles that are relevant to the analyses of samples from clinical trials while ensuring the purpose and objectives of the Good Clinical Practice principles are maintained. In so doing, the reliability, quality, consistency and https://en.wikipedia.org/wiki/Laboratory_information_management_system GxP: Acronym for the group of good practice guides governing the preclinical, clinical, manufacturing and post-market activities for regulated pharmaceuticals, biologics, medical devices, etc., such as good laboratory practices, good clinical practices, good manufacturing practices and good distribution practices..

1.1.1 Good Laboratory Practice (GLP) is a managerial concept covering the organizational process and conditions under which laboratory studies are planned, performed, monitored, recorded, archived and reported. GLP principles are required to be followed by test facilities, carrying out studies to be submitted to national authorities for the purposes of assessment of chemicals and other uses Guidelines for Good Clinical Laboratory Practices (GCLP) 3.0 LEVELS OF LABORATORIES In India, the laboratory services are integrated with the 3-tier public health system at the primary, secondary and tertiary levels. Besides these, there are Reference Laboratories, Research Laboratories and Specific Disease Reference Laboratories
Good Laboratory Practice is a quality system and the manner in which non-clinical safety studies are: Planned, performed, monitored, recorded, reported and archived. Learn the good laboratory practice regulations by taking CfPA's in-demand Good Laboratory Practices (GLP) course. This GLP training course will concentrate on OECD good laboratory iii FOREWORD In order to assist countries in conducting non-clinical research and drug development, TDR developed a Good Laboratory Practices (GLP) series in 2001, comprising a GLP Handbook as well as GLP Training manuals for trainers and trainees. The demand for this series was so substantial that it became one of the most frequent “hits”
Requirements of Schedule L1 (Indian GLP) Good Laboratory Practice in Pharmaceutical Learn about Good Laboratory Practice to be followed by the drug manufacturers in India.i.e Schedule L1. 14/06/2013 · Guidelines for Good Laboratory Practices in Life Sciences Recording 04192012 - Duration: 29 Good Laboratory Practice ( GLP ) in Hindi - Duration: 9:40. …
Good Clinical Practice; Good Laboratory Practice. Regulations and Guidelines. AGIT (Switzerland) European Medicines Agency (EMA) European Union; Environmental Protection Agency (EPA) MHRA; OECD; VICH; US FDA; Field Studies; Discussion Forum; Good Manufacturing Practice; Good Pharmacovigilance Practice; Medical Devices; Global Engagement Team In the experimental (non-clinical) research arena, good laboratory practice or GLP is a quality system of management controls for research laboratories and organizations to ensure the uniformity, consistency, reliability, reproducibility, quality, and integrity of chemical (including pharmaceuticals) non-clinical safety tests; from physio
The Good Laboratory Practice (GLP) Training Manual set comprises of two manuals; one for the trainer (red), one for the trainee (green). These have been designed for use as an introductory course to GLP. They are accompanied by a WHO/TDR Handbook on GLP (blue) which includes an introduction to GLP, texts concerning the salient points of the Good manufacturing practice contract GMP quality control testing laboratory change report (MS Word Document, 349KB) Quality control testing laboratory change report - guidelines for completion and
Good Clinical Laboratory Practice (GCLP) guidelines describe the application of those Good Laboratory Practice principles that are relevant to the analyses of samples from clinical trials while ensuring the purpose and objectives of the Good Clinical Practice principles are maintained. In so doing, the reliability, quality, consistency and Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC.
The 1981 Council Decision on the Mutual Acceptance of Data in the Assessment of Chemicals (revised in 1997) that states that test study data generated in any member country in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice (GLP) shall be accepted in other member countries for assessment purposes and other uses PDA Europe. Am Borsigturm 60 13507 - Berlin, Germany Tel: +49 30 436 55 08-0 or -10 Fax: +49 30 436 55 08-66
Benefits of good laboratory practices. • It will give better image of company as a Quality producer in Global market. • Provide hot tips on analysis of data as well as measure uncertainty and perfect record keeping. • Provide guideline for doing testing and measurement in detail. • Provide guidelines and better control for maintenance Guidelines for Good Clinical Laboratory Practices (GCLP) 3.0 LEVELS OF LABORATORIES In India, the laboratory services are integrated with the 3-tier public health system at the primary, secondary and tertiary levels. Besides these, there are Reference Laboratories, Research Laboratories and Specific Disease Reference Laboratories
good agricultural practices good field collection practices and Ayurvedic textual methods) Non clinical safety studies (5) (acute/subacute/chronic studies as per the clinical use of the drug) (with appropriate animal ethical clearances as per available guidelines ) Botanical identification/ Pharmacogonostic/Chemical studies of ingredients. (3) Directive 2004/10/EC requires EU countries to take all measures necessary to ensure that laboratories carrying out safety studies on chemical products comply with the OECD Principles of Good Laboratory Practice. Directive 2004/10/EC replaces Directive 87/18/EEC.
25/08/2010В В· Good Clinical Laboratory Practices (GCLP) should be used by all laboratories where tests are done on biological specimens for diagnosis, patient care, disease control and research. This editorial is not meant to discuss anything new but to emphasize the well accepted guidelines for GCLP. Good manufacturing practice contract GMP quality control testing laboratory change report (MS Word Document, 349KB) Quality control testing laboratory change report - guidelines for completion and

Good Laboratory Practice Ainoon Othman Department of Pathology Faculty of Medicine, UKM Fundamental points of GLP Good Laboratory Practice applied in whatever – A free PowerPoint PPT presentation (displayed as a Flash slide show) on PowerShow.com - id: 3b7e6a-OWQwM The Good Laboratory Practice (GLP) Training Manual set comprises of two manuals; one for the trainer (red), one for the trainee (green). These have been designed for use as an introductory course to GLP. They are accompanied by a WHO/TDR Handbook on GLP (blue) which includes an introduction to GLP, texts concerning the salient points of the